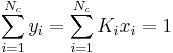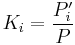Bubble point
When heating a liquid consisting of two or more components, the bubble point is the point where first bubble of vapor is formed. Given that vapor will probably have a different composition than the liquid, the bubble point (along with the dew point) at different compositions are useful data when designing distillation systems.
For single component mixtures the bubble point and the dew point are the same and are referred to as the boiling point.
Calculating the bubble point
At the bubble point, the following relationship holds:
where
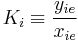 .
.
K is the distribution coefficient or K factor, defined as the ratio of mole fraction in the vapor phase 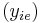 to the mole fraction in the liquid phase
to the mole fraction in the liquid phase 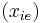 at equilibrium.
at equilibrium.
When Raoult's law and Dalton's law hold for the mixture, the K factor is defined as the ratio of the vapor pressure to the total pressure of the system:[1]
References
- ^ McCabe, Warren L.; Smith, Julian C.; Harriot, Peter (2005), Unit Operations of Chemical Engineering (seventh ed.), New York: McGraw-Hill, pp. 737–738, ISBN 0-07-284823-5